Zinc Phosphate Coating
In most operations where the corrosion resistance of finished workpieces must be especially high, conversion coatings are applied using zinc phosphate. This approach is widely used in the wire drawing works, automotive industry and in certain sectors of the appliance and electronics industries. Similarly, zinc phosphating is often specified by the armed services, especially for equipment that may be exposed to severe environments. Moreover, many operations using electrocoating or powder coatings, particularly when a one-coat finish will be exposed to the weather, pretreat workpieces with zinc phosphate.
Technical Applications Of Zinc Phosphate Coatings
- Zinc Phosphating Prior to Powder Coating
- Zinc Phosphating Prior to Oiling/Lubricating
- Zinc Phosphating Prior to Wire Drawing
- Zinc Phosphating Prior to Tube Drawing
Zinc Phosphating Prior to Powder Coating
There are countless applications of powder coating coupled with phosphate pretreatment. They include the finishing of refrigerators, pressure vessels for fire extinguishers, garden furniture, electric panels, steel fencing, car wheels and other car accessories. The degree of success in the devolopment of powder coating is largely governed by the outcome of advances in other environmentally-friendly processes such as high solids containing, water based paints or two-pack systems in the market as a whole.
Two types of phosphating are generally applied before electrostatic powder coating; iron phosphating and zinc phosphating.
The principle of powder coating is to apply the organic coating, in finely-devided powder form to the surface of the work to be coated either at ambient or somewhat elevated temperatures. The film is then formed by melting the powder on the surface of the substrate.
Powder coating is being increasingly used not least because of the absence of volatile solvents involved thus eleminating virtually all problems of airborne effluent discharge. The corrosion resistance of powder coating is many times better than of the equivalent thickness of conventional paints. Against this, it must be said that maintaining a given colour value is, by the nature of the process, more difficult for powder coating. In well-run plants using spray powder coating, the over-sprayed powder is collected and re-cycled which, if colour values are to be held, calls for high standarts of cleanliness.
Introduction of zinc phosphates and the manganese-modified zinc phosphates coupled with powder coating, allowed still further improvements as manifested in the atmospheric corrosion test, the cyclic salt spray water condensate test, and salt spray test or water condensate test. The beneficial effects of the manganese modified process are specially in evidence for galvanized steel. Even for these more recently introduced processes, the best results are found where the Cr (VI) post rinse has been used to passivate the phosphate. In certain cases, post-rinse solutions based Cr (III) or chromium-free solutions are used, the performance of which is intermediate between a simple water rinse and the Cr (VI) containin water rinse.
Zinc Phosphating Prior to Oiling/Lubricating
On account of their porosity and micro-roughness, phosphate coatings have high oil holding properties. This can be demonstrated by partly immersing blank and zinc phosphate coated steel sheet in commercially available corrosion protective oils.
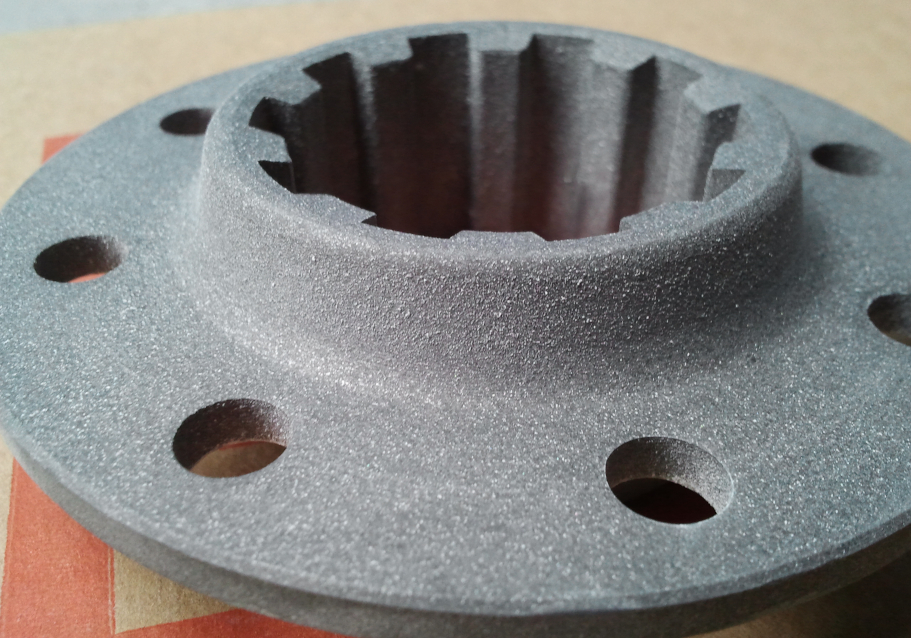
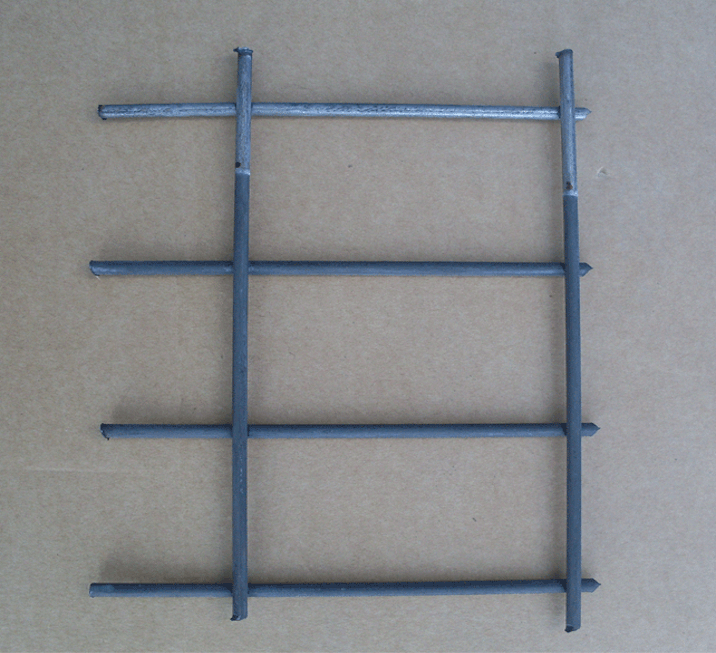
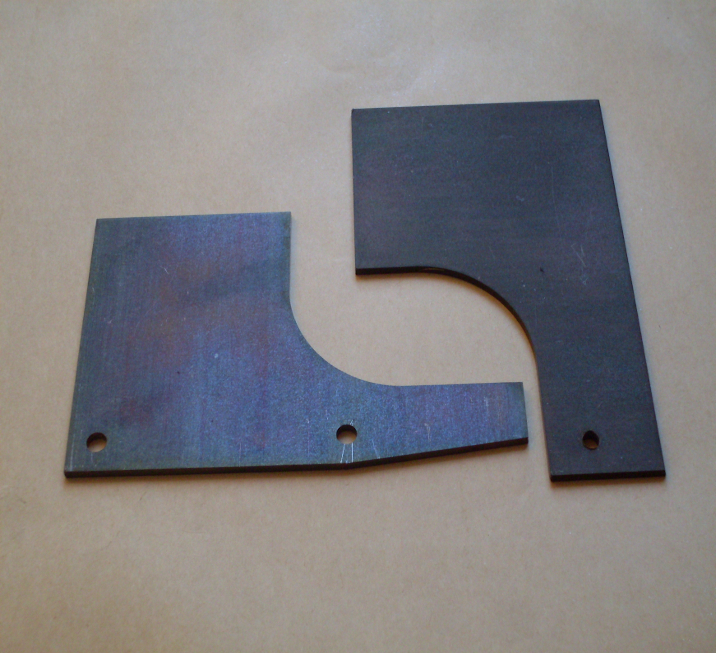
The use of such heavy phosphate coatings for improved corrosion protection can be found in nearly all branches of metal working industry. Typical examples include screws, nuts and bolts, motor vehicle components in brake and clutch assemblies, engine components, leaf or coil springs, drill bits, washers, anti-vibration washers, tools, magnet cores, casing interiors and other small items.
To ensure good corrosion resistance, phosphate coatings should not be less, than 10 g/m² except for undemanding environments where values of 5-10 g/m² are acceptable. The preferred phosphating processes are those based zinc and manganese phosphates. Zinc phosphate coatings are, in general, more corrosion resistant than those based on manganese phosphate but many users prefer manganese based ones because of darker, attractive appearance.
The type of post treatment oil or wax also affects corrosion resistance. Increase of viscosity, and thus the film thickness and its resilience, usually leads to improved resistance while equally significant are the effects of corrosion inhibitors included in the oil formulations. Surfactants, on the other hand, which are called for in the water emulsifiable oils, increase the danger of the protective oils being washed out by rain or formation of condensate.
Oils and waxes used for post treatment of phosphate coatings can be classified according to the mode of use, as follows:
- Oils and waxes dissolved in organic solvents
- Aqueous solutions of oils and waxes
- Undiluted oils
- Dewatering fluids or water-repellent oils
The main components of these oils are generally mineral oils, solvents, corrosion inhibitors and surface active agents as well as special agents to inhibit oxidation, polymerisation or bacterial action.
Corrosion protective oils, used diluted either solvents or water, are relatively viscous. Organic dilution solvents are usually low-boiling point hydrocarbons or chlorohydrocarbons. The concentrations of such oils lie in the region 5-25%. The phosphated components, after drying, are immersed in the oil baths for 0.5-2.0 minutes, allowed to drain and excess solvent evaporate. The thickness of resulting oil film depends of the oil used, its concentration and the nature of the solvent. Small parts are often barrelled or centrifuged to remove excess oil and solvent.
More important technologically, are the corrosion protective oils in aqueous emulsion. Since the parts to be treated do not first have to be dried, this approach integrates readily with the main wet processing sequence. Oil concentrations are usually 10-30% and the water should be softened since some corrosion protective oils form undesirable reaction products with salts in hard water. Drag-in electrolyte from preceding stages is also undesirable since this can affect the emulsion stability. These emulsion based systems are normally used at 60-80 ºC, sometimes above 90 ºC. Such relatively high temperatures facilitate subsequent drying of the work-pieces. Immersion times range from 0.5-2.0 minutes. Subsequent drying in a circulating air owen is only called for if the bath temperature is less than 80 ºC and there is an excess of fresh air movement. Drying at higher temperatures reduces the thickness of the oil film and thus the corrosion resistance.
Zinc Phosphating Prior to Wire Drawing
The primary benefit in phosphating of wires and sections for drawing and profiling lies in its superb adhesion and the survival of the coating even after several passes through a die. The crystalline nature of the phosphate coating offers an excellent substrate for the lubricant used, inhibiting film rupture of these.
Use of phosphated steel reduces wear of tools and dies and, in practice, significant increases in the working life of the diesis found. The phosphate film after drawing imparts a high surface finish to the wire at the same time affording a certain degree of corrosion protection during storage and transport.
In case of hot dip galvanized iron or steel wires which are then further drawn, phosphating has advantages to offer. With a heavy zinc coating of 80-150 g/m², there is a tendency for such wires to build up debris in the drawing die which results in increased wear rates of the die, a reduction in the quality of the drawn wire surface and the failure of brakes in the drawing operation. By phosphating in conjunction with galvanizing, all these problems are largely eliminated. The wire product has a somewhat darker appearance, but is ductile with a smooth, corrosion resistant surface.
In the production of thin, low carbon wires in a drawing machine, phosphating can increase the production rate while at the same time providing corrosion protection during storage and transport.
In drawing of phosphated steel wires, care must be taken to use correct phosphate coating thickness. If it is too great, enhanced friction will occur at the first drawing, resulting in bloom on the wire. On the other hand, though, the phosphate coating must be sufficiently which that its benefits persists even at the last of a number of drawings. As a general rule, for multiple drawing of high carbon wires, a residual phosphate film after the final drawing of 0.5 - 1.0 g/m² is desirable. Exceptions to this are those steel wires where a phosphate film is useful in some subsequent forming process. Thus wires for cold extrusion and cold forging require phosphate coatings of 5-15 g/m² as measured after the first calibrating pass.
Immediately before immersion phosphating of wire coils, an alkaline pre-rinse is often installed. This serves to neutralise residual acid traces on the incoming material. Such alkaline pre-rinses with added activating agents such as titanium salts have proved especially valuable in their promotion of very fine dense zinc phosphate coatings. Such finely crystalline coatings are less liable to lead to bloom on drawn wire than their coarser-crystalline forms.
Phosphating of wires is usually based on the immersion method in which coils of wire are lowered into individual baths. The phosphating tank is thus incorporated together with other post-treatment stages downstream of the pickling plant. A typical treatment sequence for wire bundles can be schematically shown as follows:
- Pickling, in several stages, with sulphuric acid or hydrochloric acid
- Water rinse, for example with two dip tanks and a spray rinse
- Activation
- Phosphating
- Water rinse
- Post treatment
- Drying
Zinc Phosphate Coating Prior to Tube Drawing
Where phosphating is used for the cold drawing of seamless tube, the requirements are somewhat different from those applicable to welded steel tube. Until about 30 years ago, phosphate coating weights of 20-40 g/m² were used allowing the largest possible number of successive draws. Such coatings were formed with nitrate-accelerated systems at 90-95 °C.
Over the years, in search for increased operational efficiency, the number of draws for a given reduction of seamless tubing, has decreased. In order to maintain the dimensional tolerances and quality of surface finish, tubes in the mid-range of available sizes are now reduced in a single or, at most, two draws. At the same time, drawing rates have been increased. For these reasons phosphate coating for drawing of seamless tubing are now formed with weights of 4-10 g/m². This has improved the efficiency of the surface treatment and, at the same time, avoided the adverse effects which act in the firs drawing stage where coarser-crystalline phosphate coating are found. The most suitable coating is based on nitrate/nitrite accelerated zinc phosphate, formed at 40-75 °C. At the upper end of this temperature range, the option exists to use self dosing nitrate type systems. Chlorate accelerated zinc phosphate baths are also found. In all cases, the preferred form of the phosphate for cold drawing of tube and section is strongly adherent but soft structured.
In the drawing of welded tubing, the seam must first be ground down. In the case of smaller diameter tubing, this is not possible inside the welding machine. In some cases, there may be a deformation to give a particular cross-section. Since, as a rule, less severe deformations can be tolerated by welded, as opposed to seamless tubing, the use of phosphating is widespread, coating weights being of the order 1.5 - 5 g/m². These are mostly based on zinc phosphate baths operated between 50 and 75 °C with additives used to promote thinner coatings.
Phosphating is also used for tubing of un-alloyed or low-alloyed steel with chromium content up to 4-6%. Such coatings offer a number of advantages, all arising from reduced metal-to-metal contact between tube and die. Thus, cold welding damage, leading to grooving or crack formation, is minimised, tool and die life is extended and higher drawing rates may be used. Zinc phosphate coating also allows a greater degree of reduction per pass and an increased number of passes without intermediate heat-treatment.
Phosphating is carried out by immersion along the following lines:
- Alkaline degreasing.
- Water rinse.
- Pickling in sulphuric or hydrochloric acid.
- Water rinse.
- Neutralising pre-rinse.
- Phosphating.
- Water rinse.
- Neutralising rinse.
- Lubrication.
- Drying and storage.
In earlier times, the surface treatment of tubes took place using bundled tubes lifted by a manually-operated hoist from one tank to the next. More recently, automatic conveyors have been introduced and, while retaining the sequence of operations listed above, the whole operation takes place automatically.
Application Methods Of Zinc Phosphate Coatings
Immersion Process
In the immersion mode, zinc and alkali metal phosphating systems do not greatly differ from one other. The individual steps, as normally carried out are:
| 1 | Cleaning Stage | Temp.: 55-95 °C | Time: 5-10 min. |
| 2 | Water Rinse | Temp.: 15-30 °C | Time: 0.5-1.5 min. |
| 3 | Activation | Temp.: 20-40 °C | Time: 0.5-1.5 min. |
| 4 | Phosphating | Temp.: 40-60 °C | Time: 3-10 min. |
| 5 | Water Rinse | Temp.: 15-30 °C | Time: 0.5-1.5 min. |
| 6 | Post-Rinse | Temp.: 20-40 °C | Time: 0.5-1.5 min. |
Cleaning is usually based on alkaline or strongly alkaline solutions in the concentration range 1-5%, and pH 10-13. Compared to the cleaners used in the spray mode, the cleaners used for immersion contain larger amounts of silicate and sodium hydroxide. The surfactants used often include the strongly-foaming anionic types, usually mixed with non-ionic types.
Activation is mandatory for zinc phosphating and in its absence, because of the relatively high pH values involved, a thick and coarse-crystalline coating is formed which is quite unsuited of subsequent painting. Conditions for the rinse, post-rinse and drying stages differ little from spray phosphating.
Spray Process
In case of spray phosphating, major differences are found in the process sequences for zinc phosphating and alkali metal phosphating systems.
Zinc phosphating lines are normally based on 5-stage to 6-stage plants. They differ little in the pre-phosphating stages. The 5-stage type includes a rinse between cleaning and phosphating steps. In the case of the 6-stage type, there are either two cleaning and one rinse stages or one cleaning and two rinsing steps.
| 1 | Cleaning | Temp.: 40-60 °C | Pressure: 1.0-2.5 bar | Time: 2.0-2.5 min. |
| 2 | Water Rinse | Temp.: 15-30 °C | Pressure: 0.7-1.5 bar | Time: 0.5-0.7 min. |
| 3 | Activation | Temp.: 15-30 °C | Pressure: 0.7-1.5 bar | Time: 0.5-0.7 min. |
| 4 | Phosphating | Temp.: 40-60 °C | Pressure: 1.0-2.0 bar | Time: 2.0-2.5 min. |
| 5 | Water Rinse | Temp.: 15-30 °C | Pressure: 0.7-1.5 bar | Time: 0.5-0.7 min. |
| 6 | Passivation | Temp.: 20-40 °C | Pressure: 0.7-1.5 bar | Time: 0.5-0.7 min. |
The cleaning stage is usually based on weakly alkaline products based on alkali phosphates, alkali carbonates or borates, low-foaming surfactants and alkali silicates. To these, further compounds such as titanium orthophosphates may be added to increase the rate of the phosphating process and facilitate formation of uniform finely-crystalline coatings.
It is normal to dry phosphated work before the application of conventional paints and in the case of anodic electro coating paint, it is highly recommended. Circulating air ovens, operating at 120-180 °C with a drying time of 5-15 minutes are typically used for such drying.
In 4-stage plants the concentrations in the first bath run from 3-10 g/l, somewhat higher (5-15 g/l) in the second bath. The rinse and post-rinse stages are operated on the same basis as normal zinc phosphating systems.